The University of Liverpool’s discovery of a new lithium-ion conductor represents a pivotal advancement in battery technology, offering the potential for safer and more energy-efficient batteries. This innovation, stemming from the synthesis of non-toxic, earth-abundant elements, could revolutionize the way electronic devices and electric vehicles are powered. By leveraging AI and collaborative scientific methodologies, researchers have expanded the chemical space for future discoveries, underscoring the vital role of AI in the evolution of material science. This development not only signifies a leap towards sustainable energy solutions but also highlights the transformative potential of integrating artificial intelligence in the quest for groundbreaking materials.
What’s new in this Lithium-ion discovery?
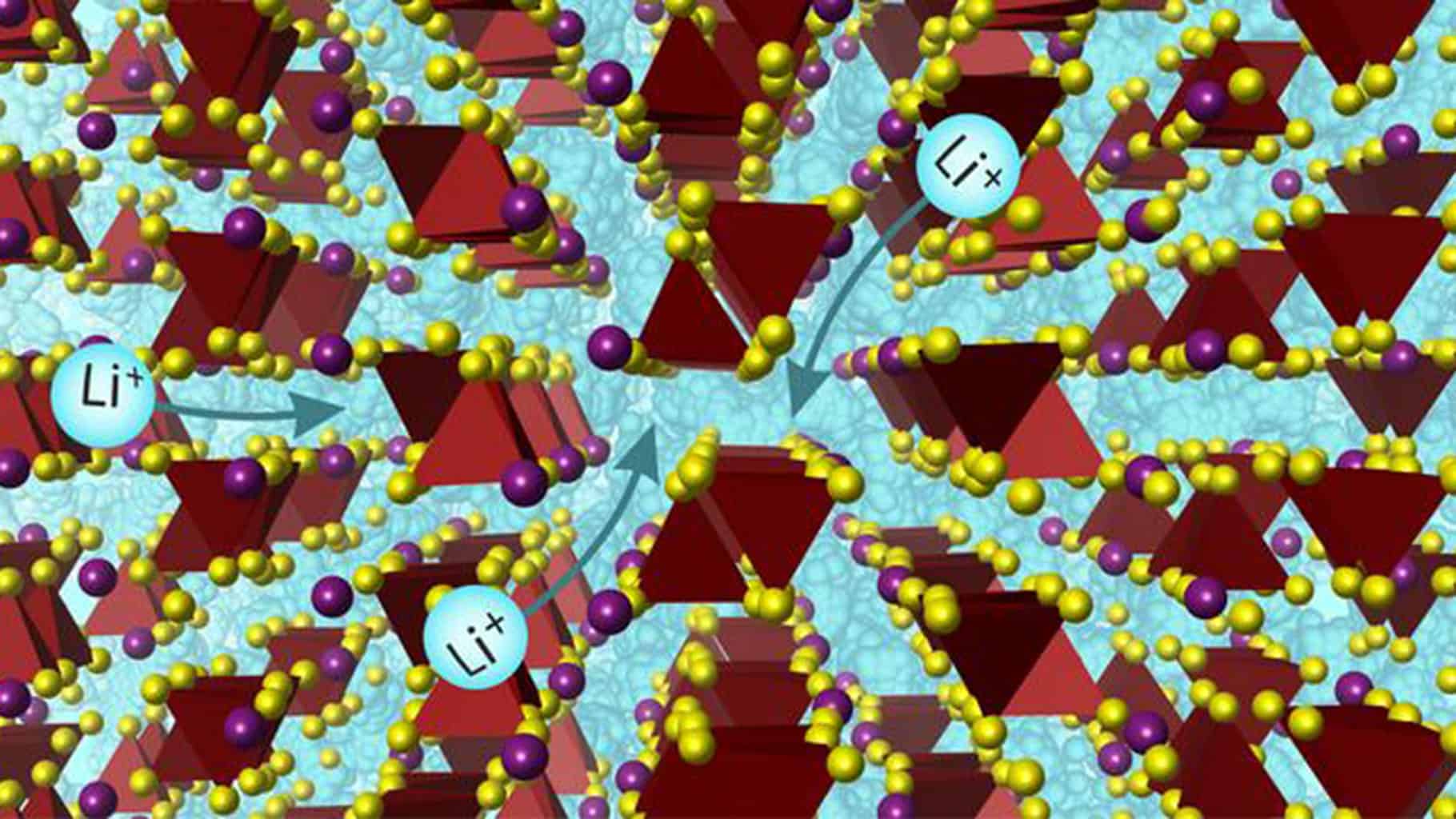
Researchers at the University of Liverpool have made a significant breakthrough in battery technology by discovering a strong substance that serves as a quick lithium-ion conductor. This new material, composed of non-toxic, earth-abundant elements, boasts lithium-ion conductivity sufficient to potentially replace wet electrolytes in the latest Lion battery technology. This advancement promises to enhance both the safety and energy efficiency of batteries.
A Leap Towards Safer and More Efficient Batteries
These lithium electrolytes are crucial components of rechargeable batteries, powering a plethora of electronic devices and electric vehicles. Published in the journal Science, the study titled “Superionic lithium transport via several coordination environments defined by two-anion packing” explores the possibility of substituting liquid electrolytes with this solid lithium-ion conductor. The material was meticulously synthesized in the laboratory, its structure thoroughly analyzed, and its effectiveness demonstrated in a battery cell. The innovative scientific methods employed in its design mark a significant step forward in battery technology.
The Structural Advantage
What sets this new substance apart is its exceptional lithium-ion conductivity, making it one of the few materials capable of replacing liquid electrolytes effectively. Its unique structure enables it to function distinctly from its counterparts. The discovery process utilized a collaborative approach that combined artificial intelligence (AI) and physics-based calculations, guided by the expertise of University of Liverpool’s chemistry professionals. This method underscores the potential of AI in advancing material science, particularly in identifying and optimizing novel materials.

Exploring New Frontiers with AI
This groundbreaking study not only showcases the design and discovery of a material that is both innovative and practical but also redefines the understanding of high-performance solid-state electrolytes. According to Professor Matt Rosseinsky of the Chemistry Department at the University of Liverpool, the diverse ionic environments within the solid contribute to its superior performance, expanding the possibilities for future material discoveries.
The Role of AI in Material Discovery
The utilization of AI tools in discovering potential new materials has gained traction, as evidenced by recent reports and media coverage. These tools, operating autonomously, are adept at replicating their training across various contexts, thereby identifying materials with potential similarities to known ones. This research paper highlights how AI, when combined with expert knowledge, can address the complex challenge of discovering real-world materials with unique compositions and structures, significantly impacting their properties.
The innovative design approach outlined in this study paves the way for the discovery of high-performance materials reliant on the rapid movement of lithium ions in solids. This breakthrough not only enhances our understanding of solid-state electrolytes but also opens up new avenues for the development of safer, more energy-efficient battery technologies.