In the realm of electric vehicles and portable electronics, the proliferation of lithium-ion batteries has brought with it concerns over safety, particularly regarding the risk of battery fires. Addressing this pressing issue, a groundbreaking study introduces a novel approach to battery design, utilizing components from commercial fire extinguishers to create self-extinguishing batteries. This innovative solution not only mitigates the risk of lithium-ion battery fires but also enhances overall safety and durability. By replacing conventional combustible electrolytes with nonflammable alternatives derived from professional coolants, the study pioneers a paradigm shift in battery technology.
Bingan Lu, an associate professor of physics and electronics at Hunan University, and Apparao Rao, physicists at Clemson University discuss the topic
As electronic vehicles become more widespread, lithium-ion battery fires are becoming more frequent and difficult to put out. A brand-new method makes use of an electrolyte and a corporate fire extinguisher
From about minus 100 to 175 degrees Fahrenheit ( minus 75 to 80 degrees Celsius ), our electrolyte performed well at a variety of temperatures. With the help of this electrolyte, we created batteries in the lab that essentially burned interior fires and transferred heat aside from the battery.
We tested these batteries using the nail penetration test, a popular technique for determining the safety of lithium-ion batteries. A charged battery is driven through a stainless steel nail to simulate an inner little circuit; if the battery catches fire, the test fails. Our charged batteries withstood the impact when we drove a nail through them without catching fire.
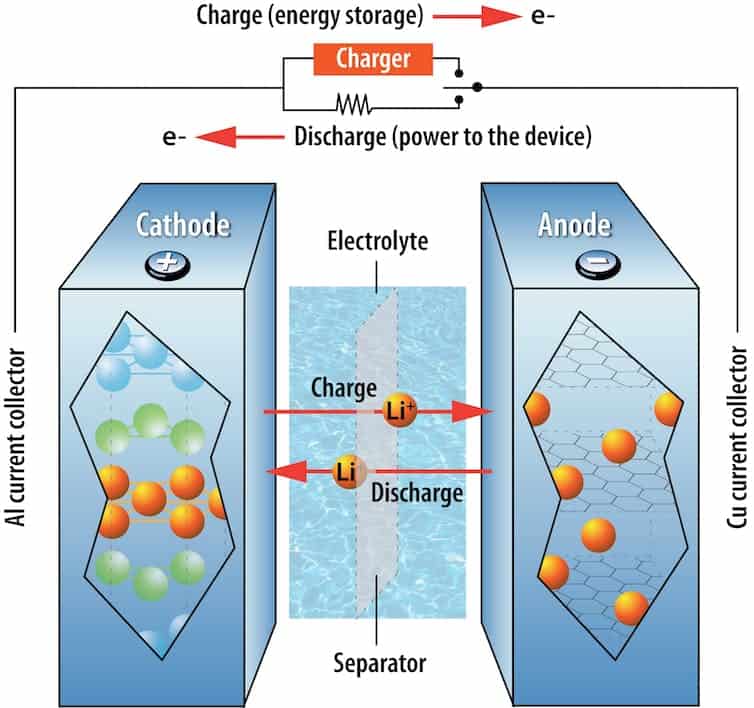
Why do Battery Safety matters?
According to domestic resistance, or resistance within the battery to the flow of lithium ions, a battery’s temperature naturally changes as it charges and discharges. The safety and durability of batteries are seriously jeopardized by great outdoor temperatures or uneven temperatures inside a battery pack.
Energy-dense batteries, like the lithium-ion models that are frequently used in electronics and electric vehicles, have an liquid formulation that is dominated by highly volatile organic molecules. This increases the risk of thermal fugitive, an uncontrollable process in which unwelcome chemical reactions are accelerated by excessive heat inside a battery and cause additional reactions. A fire or explosion can result from temperatures inside the battery rising by hundreds of degrees in a split second.
When lithium-ion batteries are charged to swiftly, another safety issue arises. On the battery’s anode, the electrode with a bad charge, this can result in chemical reactions that produce extremely strong lithium needles known as dendrites. Overheating results from the needles gradually passing through the separator and reaching the other electrode, short-circuiting the battery internally.
We have a keen interest in creating energy-dense and secure batteries as scientists studying energy generation, storage, and conversion. Lithium-ion batteries may become safer if volatile electrolytes are swapped out for a flame-retardant one. This could buy time for longer-term improvements that would lessen the risks of burning and thermal runaway.
How we performed the work on Self-extinguishing batteries
We sought to create an electrolyte that was nonflammable, easily transferred heat from the battery pack, could operate at a variety of temperatures, was extremely tough, and would work with any battery chemistry. The majority of well-known nonflammable natural solvents, however, are expensive and have the potential to harm the environment because they contain fluorine and phosphorus.
Instead, we concentrated on making inexpensive business coolants that were already widely used as battery electrolytes in fire extinguishers, digital testing, and cleaning applications.
We concentrated on Novec 7300, a intelligent, secure, and reasonably priced commercial fluid that has low toxicity, is nonflammable and does not cause global warming. We were able to create an electrolyte that had the properties we were looking for and would allow a battery to charge and discharge for an entire year without significantly losing capacity by combining this flow with various other chemicals that added durability.
What is still unknown about Lithium-ion batteries
It is crucial to look into how nicely batteries that use another, more plentiful alkaline metal ions, such as potassium or sodium, fare in comparison because lithium, an alkaline metal, is limited in our planet’s crust. Because of this, the majority of our research was on self-extinguishing potassium ion batteries, though it also demonstrated that our electrolyte is effective at producing lithium ions that can also be put out on their own.
It is yet to be seen if our electrolyte can function just as well for another battery types that are currently being developed, like sodium-ion batteries, aluminum-ions, and zinc ions batteries. Regardless of the type of ion, our objective is to create useful, environmentally friendly, and long-lasting batteries.
However, for the time being, our alternative electrolyte can be easily integrated with existing battery production lines because it has physical properties that are comparable to those of presently used electrodes. We anticipate that businesses will be able to produce nonflammable batteries using their current lithium-ion battery facilities if the industry adopts it.










